The medical device industry is one of the most tightly regulated sectors in the world and for good reason. When products directly affect patient health and safety, compliance with strict standards isn’t optional. It’s essential.
For job seekers, particularly those entering roles in quality, regulatory affairs, R&D, or manufacturing, understanding medical device regulations such as EU MDR (Medical Device Regulation) or ISO 13485 can make the difference between getting hired or being passed over.
In fact, in today’s competitive life sciences job market, having foundational knowledge in medical device regulatory frameworks is becoming a key employ-ability asset—and not just for regulatory affairs roles. Let’s explore why.
Employers Want Job-Ready Candidates
Companies in the medtech and biotech sectors often operate under strict timelines and resource constraints. Whether a startup or a multinational, hiring managers don’t just want smart people, they want team members who can hit the ground running.
This is especially true in regulated environments, where employees must work within established procedures, document everything accurately, and understand the implications of non-compliance.
Hiring someone with no knowledge of medical device regulations means the company has to:
- Provide extra training (which costs time and money)
- Monitor for avoidable errors or missteps
- Delay important tasks while onboarding is completed
On the other hand, a candidate who already understands the basics of CE marking, clinical evaluation reports, post-market surveillance, or design controls brings immediate value.
A Real-World Hiring Perspective
Talk to hiring managers in medtech and biotech, and a consistent message emerges:
“We get many technically qualified candidates, but very few understand the regulatory environment. That’s often the deciding factor.”
“We don’t have the internal bandwidth to train junior hires from scratch. If they know what MDR or risk classification is, that’s a huge plus.”
regulatory awareness is no longer a niche skill—it’s a baseline expectation, especially in Europe where MDR has raised the bar for compliance across the board.
Understanding MDR: A Must for EU Professionals
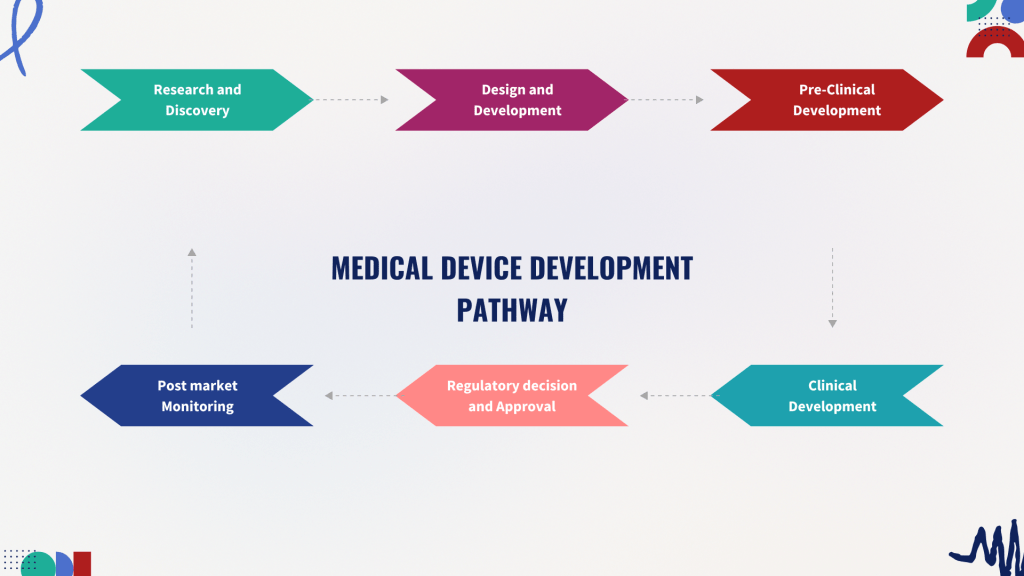
The 2021 implementation of the EU Medical Device Regulation (MDR) fundamentally changed how medical devices are developed, evaluated, and marketed in Europe.
Key changes include:
- Stricter clinical evidence requirements
- Tighter classification rules
- More extensive documentation and technical files
- New obligations for post-market surveillance
- Extended responsibilities for manufacturers and economic operators
For employers, navigating MDR means maintaining compliance while adapting to evolving expectations. Every function—R&D, QA/RA, manufacturing, even marketing must understand the new landscape.
If you’re applying for any role touching medical devices in the EU, understanding MDR is not optional it’s a professional necessity.
How Regulatory Knowledge Sets You Apart
Here’s how being educated in medical device regulation gives you a clear edge:
1. Shortens Your Onboarding Time
Employees who already understand regulatory concepts require less internal training and can start contributing sooner, making you more attractive to employers.
2. Reduces Risk for Employers
New hires unfamiliar with regulations can inadvertently make costly mistakes, such as failing to document correctly or violating quality procedures. Your knowledge reduces compliance risk.
3. Shows Initiative and Professional Maturity
Being proactive in learning about regulation demonstrates that you’re serious about your career in medtech or biotech and understand the bigger picture beyond the lab or product design.
4. Enables Cross-Functional Communication
Understanding the regulatory context helps you collaborate more effectively with QA/RA teams, design engineers, clinical departments, or notified bodies.
5. Improves Interview Performance When you can reference MDR classification rules, post-market requirements, or ISO 13485 clauses in an interview, it signals depth and instantly differentiates you from other candidates.
It’s Not Just for Regulatory Affairs Roles
You might think medical device regulations are only relevant if you’re working in QA/RA. Not true. Here’s why professionals in other areas should care:
- R&D and Engineering
Need to design products in line with design control, risk management, and usability engineering requirements. - Clinical Research
Must understand requirements for clinical evaluation, PMS, and post-market clinical follow-up (PMCF). - Manufacturing and Quality
Deal with CAPAs, internal audits, change control, and supplier qualification under ISO 13485 or MDR requirements. - Marketing and Product Management
Must know what claims can legally be made, how labeling must be managed, and how to support compliance in promotional materials.
Knowing regulations doesn’t make you less creative or flexible. In fact, it empowers you to make better, more compliant decisions faster.
How You Can Build This Knowledge (Quietly)
If you’re a job seeker in biosciences, medtech, or related fields, you don’t need to become a regulatory expert overnight. But having working knowledge of key principles and terminology is essential.
Start by learning about:
- What counts as a medical device under MDR
- The different risk classes (I, IIa, IIb, III)
- What CE marking involves
- Basic ISO 13485 principles
- The concept of a “technical file” or “clinical evaluation”
This level of knowledge will already place you in the top 10% of candidates for many roles. And if you’re building your own learning platform or course? This is exactly the kind of gap you’re aiming to fill—bridging academic training and real-world regulatory knowledge
Final Thoughts
As the medical device industry continues to grow—and regulatory scrutiny increases—employers will increasingly favor candidates who come prepared.
Having solid knowledge of medical device regulation:
- Makes you easier to hire
- Reduces the training burden on companies
- Positions you as a more valuable team member
- Opens doors to more specialized, higher-paying roles
Whether you’re a biomedical graduate, lab researcher, or aspiring QA/RA professional, now is the time to invest in your regulatory understanding. In a competitive job market, it’s no longer a bonus it’s a strategic career move.
